
Without constant input of energy, the room would eventually be quite disorganized (this isn’t strictly the same thing but is an interesting parallel).Īll processes have only two ways to transfer or change internal energy Our rooms are rarely in perfect order unless we expend energy to keep it so. We can see an analogy of this in our day to day lives in non scientific ways as well. The second law, thus, is a statement describing the driving force of all spontaneous processes. ( )įor any spontaneous process (closed or not) the entropy of the universe must increase. įor any spontaneous process in a closed system the entropy of the system must increase. Entropy is the scientific name for the macroscopic measure we use to evaluate these microstates of the system and is given the symbol. Clearly, there are more microstates available to the system if the gas expands into both chambers. A given instantaneous combination of position and energy of all the molecules taken together is called a microstate. The driving force seems to be an increase in freedom of motion of the individual molecules. This increase in room allows more freedom of motion for the individual gas molecules. The gas has more room in the increased volume of the double bulb container. The process is obviously spontaneous but energy is not involved. Gas initially in the chamber on the left (shaded) expands adiabatically into the evacuated second chamber (adiabatic means no energy transfer between system and surroundings). To start our thought experiment, we open the valve. The diagram on the left depicts an insulated, closed system consisting of two interconnected chambers separated by a valve. Back to Top 15.2 Entropy and the Second law of Thermodynamics To understand the driving force, we must introduce a new concept.

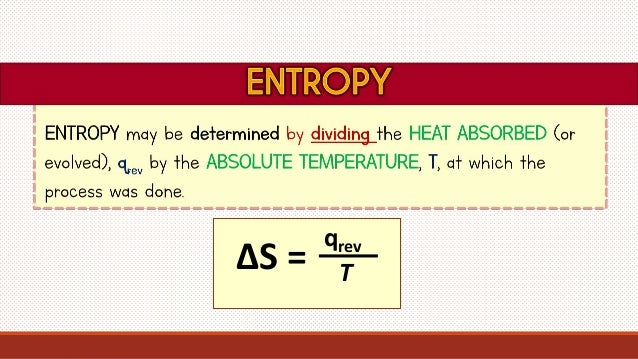
It turns out that the First Law of Thermodynamics completely eliminates enthalpy alone as a driving force Although we will see later that it does have a place in the overall equations to determine spontaneity. Equation (2) is endothermic and so there is some other driving force which pushes the reaction. If we look at these two reactions, we have difficulty deciding anything except that enthalpy change alone is not sufficient to decide if a reaction is spontaneous or not.Įquation (1) is very exothermic and this in itself is a strong tendency towards spontaneity. Obviously, the evolution of enthalpy does not drive a reaction as one might first expect. If we look at some processes which we know to be spontaneous, maybe we can find a common trend, or at least eliminate some possibilities.Ĭonsider The following two spontaneous reactions: What is the driving force that creates spontaneity? How do we measure it? We’ve seen from experience that some chemical (and other) processes are spontaneous while others are not.

Michael Mombourquette 15.1 Spontaneous Processes 15 Thermochemistry II Spontaneity, Entropy and Gibbs Energy


 0 kommentar(er)
0 kommentar(er)
